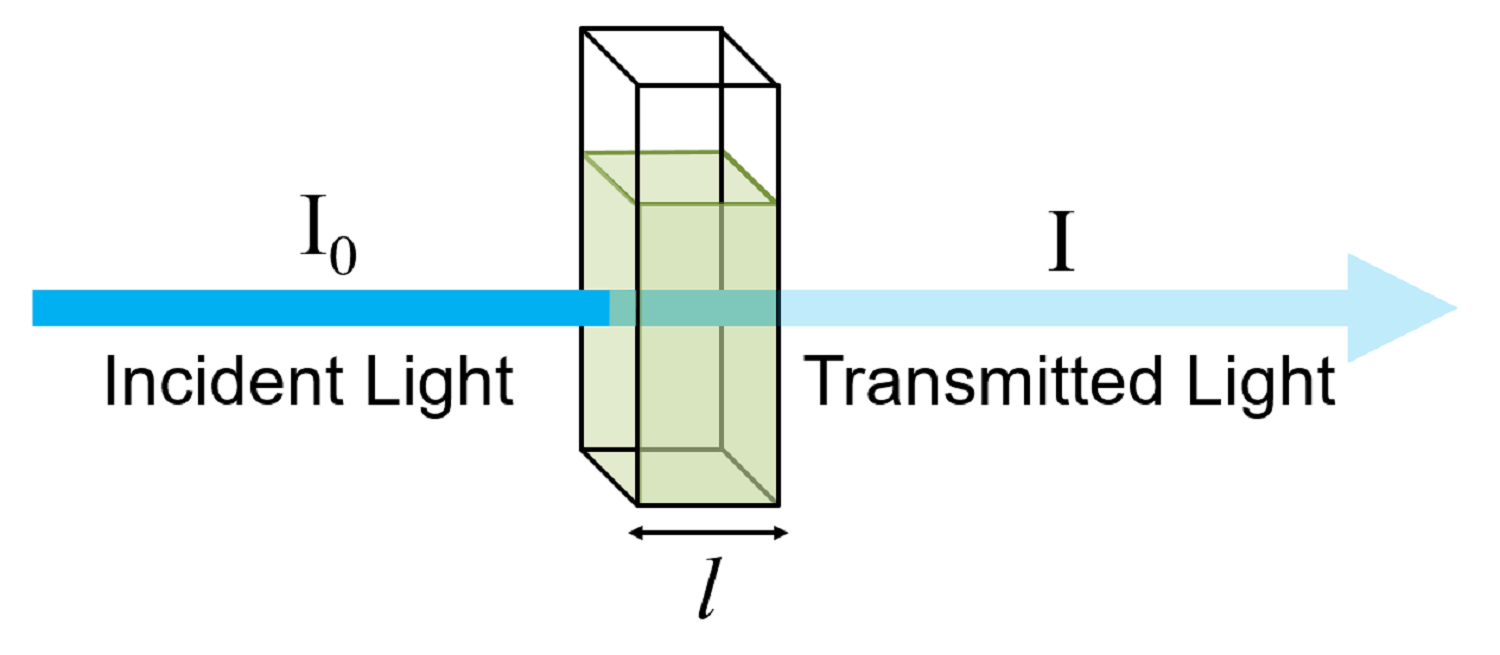
Beer Lambert Law Curve . since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. You can also use this. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law.
from www.edinst.com
You can also use this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law.
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments
Beer Lambert Law Curve since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. You can also use this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law.
From studylib.net
Beer Lambert`s law, Raman spectroscopy Beer Lambert Law Curve since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. You can also use this. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer Lambert Law Curve.
From cewoyibk.blob.core.windows.net
Beer Lambert Law Path Length at Kerry Vargas blog Beer Lambert Law Curve equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. You can also use this. Beer Lambert Law Curve.
From www.chegg.com
Solved Calculating concentration of an unknown using beer Beer Lambert Law Curve You can also use this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer Lambert Law Curve.
From www.youtube.com
Beer's Law Overview YouTube Beer Lambert Law Curve since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. You can also use this. Beer Lambert Law Curve.
From www.purechemistry.org
BeerLambert Law Purechemistry Beer Lambert Law Curve You can also use this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer Lambert Law Curve.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer Lambert Law Curve You can also use this. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer Lambert Law Curve.
From www.egpat.com
Deviations from BeerLambert law Beer Lambert Law Curve You can also use this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer Lambert Law Curve.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 Beer Lambert Law Curve since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. You can also use this. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer Lambert Law Curve.
From www.purechemistry.org
BeerLambert Law Purechemistry Beer Lambert Law Curve You can also use this. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer Lambert Law Curve.
From www.researchgate.net
Calibration curve according to BeerLambert equation for tetracycline Beer Lambert Law Curve equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. You can also use this. Beer Lambert Law Curve.
From cecgpfbn.blob.core.windows.net
Beer Lambert Law Dna Concentration at Mack Moss blog Beer Lambert Law Curve equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. You can also use this. Beer Lambert Law Curve.
From edu.rsc.org
Smartphone spectroscopy BeerLambert law Resource RSC Education Beer Lambert Law Curve You can also use this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer Lambert Law Curve.
From cienciaydatos.org
Principio de absorción espectroscópica de BeerLambert Beer Lambert Law Curve equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. You can also use this. Beer Lambert Law Curve.
From chemistrytalk.org
BeerLambert Law ChemTalk Beer Lambert Law Curve since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. You can also use this. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer Lambert Law Curve.
From stuff.iorodeo.com
Lab 2 Beer’s Law and Molar Extinction Coefficients — Colorimeter User Beer Lambert Law Curve You can also use this. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer Lambert Law Curve.
From slidetodoc.com
Beers Lambert Law and Standard Curves BCH 312 Beer Lambert Law Curve You can also use this. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. Beer Lambert Law Curve.
From www.researchgate.net
Fitting of light attenuation curve by LambertBeer model (a) and Beer Lambert Law Curve equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. You can also use this. Beer Lambert Law Curve.
From chem.libretexts.org
8.2 Beer's Law Chemistry LibreTexts Beer Lambert Law Curve equation 8.2.3 and equation 8.2.4, which establish the linear relationship between absorbance and concentration, are known as beer’s law. since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. You can also use this. Beer Lambert Law Curve.